Abstract
Aims
To evaluate the safety and efficacy of anti-CD7 chimeric antigen receptor-T (CAR-T) therapies in teenagers and adult patients with relapsed/refractory (R/R) T cell malignancies, as well as differences between autologous and allogeneic CAR-T cells.
Methods
This Phase I, single-arm, open-label clinical trial (NCT04823091) enrolled patients with R/R T-Cell Acute Lymphoblastic Leukemia (T-ALL)/ Lymphoblastic Lymphoma (LBL) (aged 14-70 years). Patients underwent lymphodepletion with fludarabine and cyclophosphamide followed by CAR-T infusion at a dose of 1x106 or 2x106/kg on D0. CAR-T cells were manufactured either from patients or donors, based primarily on the patient's tumor burden, pancytopenia status, donor availability and the wishes of the patient. Donors could be 5/10 HLA-identical siblings or 10/10 HLA-matched. Bridging therapies were allowed if disease progressed between infusion and the collection of peripheral blood mononuclear cells (PBMCs).
Results
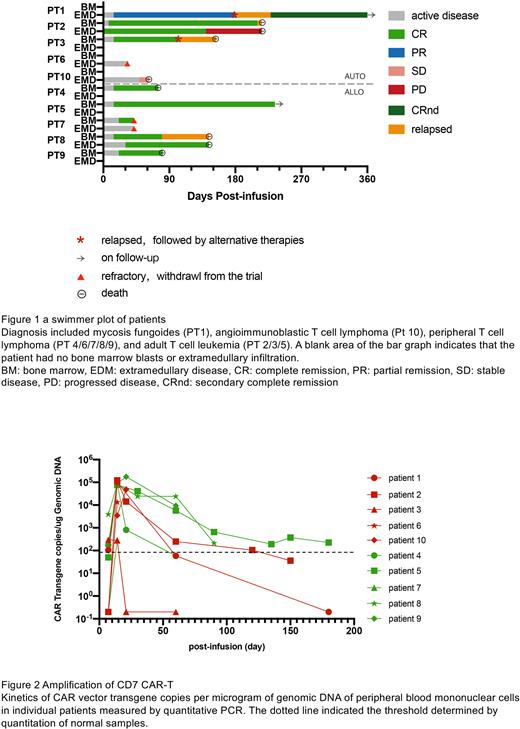
From June 2021 to July 2022, 10 patients aged 14 to 69 with T-ALL (N=3) and T-LBL (N=7) were treated with anti-CD7 CAR-T cells. Five patients received autologous CAR-T cells and five received allogeneic CAR-T cells. Seven patients experienced grade ≤2 cytokine release syndrome (CRS) and one developed grade 3 CRS. The median onset and duration of CRS was 10 days and 4 days, respectively. Immune effector cell-associated neurotoxicity syndrome was not observed. Grade 1-2 graft versus host disease(GVHD)occurred in two patients. Patient (PT) 4 who receive allogeneic CAR-T developed acute GVHD presenting as diarrhea and maculopapular rash. PT2 with previous allogeneic transplantation received autologous CAR-T cells and developed chronic GVHD characterized as skin desquamation and pigmentation. Nine infections occurred in 5 patients, of which 55.6% occurred within 1 month after infusion.
The overall response rate (ORR) was 80%. The complete response (CR) rate of bone marrow was 100% (7/7) and ORR of extramedullary infiltration was 40% (CR: n=1, PR: n=1). With a median follow-up of 5 months (3-14 months), PT5 with T-ALL maintained MRD-negative remission for 9 months and PT1 with mycosis fungoides achieved complete remission after low-dose local radiotherapy with no relapse to date (Figure 1).
The median time of CAR-T peak expansion was 14 days after infusion (7-23 days) and the median peak numbers of CAR-T proliferation measured by flow cytometry and qPCR were 4.09x107/L (1.14x106-8.64x108/L) and 7.95x104 copies/ug DNA (2.88x102 -1.75x105 copies/ug DNA), respectively. Based on the statistical analysis with a small sample size, the peak number was not significantly correlated with the patients’ cell sources, disease subtypes, tumor burden or dose of infused cells, but was associated with the best efficacy (P=0.02). During follow-up, 50% of patients (4/8) had a relatively high level of CAR-T detected by qPCR at month 2, among whom 3 received allogeneic CAR-T cells and 1 received autologous CAR-T cells (Figure 2).
Although there were no significant differences between autologous and allogeneic CAR-T cells in terms of peak expansion and clinical response at month 1, allogeneic CAR-T cells exhibited significant longer duration. Patients receiving allogeneic CAR-T have a lower recurrence rate (25% vs 100%). As for safety, the incidence of adverse events (autologous vs allogeneic, severe CRS: 20% vs 0%, GVHD: 20% vs 20%, infection in one month: 60% vs 20%) was not increased in the allogeneic CAR-T group in 1 month. However, longer duration of thrombocytopenia (median: 25 vs 18 days) and virus infections (40% vs 20%) were more common in allogeneic CAR-T group, which might lead to the death of PT9 due to cerebral hemorrhage or PT4 due to viral pneumonia.
Conclusion
These results indicate that anti-CD7 CAR-T is a promising option for teenager and adult patients with R/R T cell malignancies, but it is crucial to choose the source of CAR-T cells and the corresponding support treatment. Due to the higher relapse rate in patients receiving autologous CAR-T cells, we recommend consolidation therapy should be considered. For patients with allogeneic CAR-T cells, long-term monitoring and timely intervention for hemogram and infection may significantly improve patients’ outcomes. Larger sample size and longer follow-up are needed to confirm this conclusion.
Disclosures
No relevant conflicts of interest to declare.
Author notes
Asterisk with author names denotes non-ASH members.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal